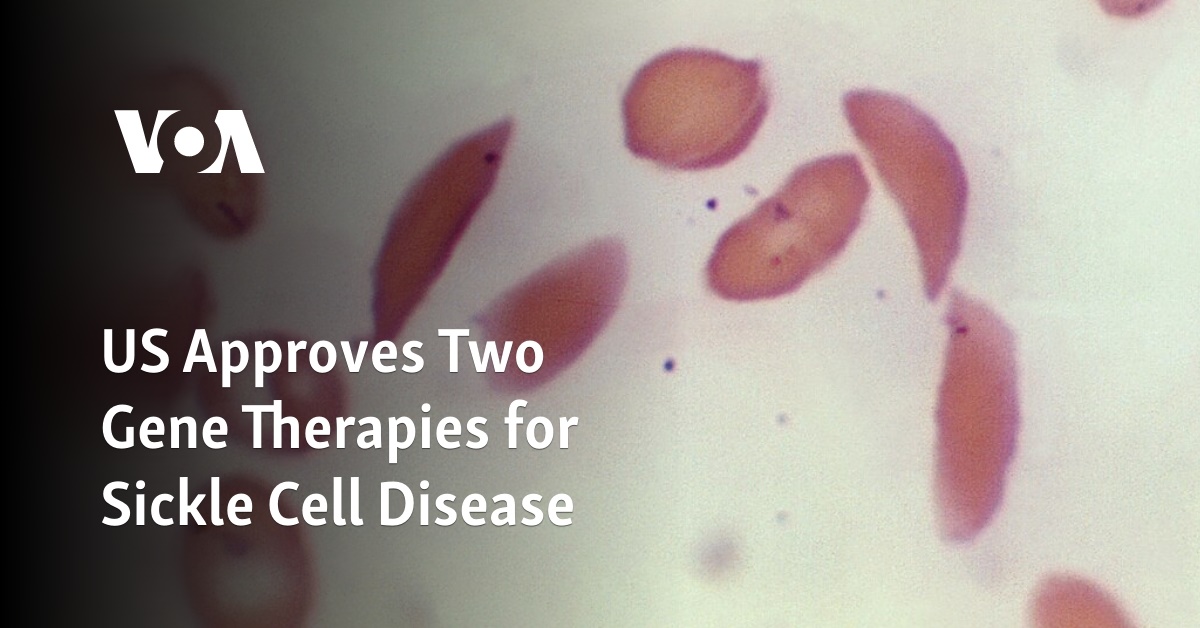
US Approves Two Gene Therapies for Sickle Cell Disease
The U.S. Food and Drug Administration (FDA) on Friday approved a pair of gene therapies for sickle cell disease, including the first treatment based on the breakthrough CRISPR gene editing technology. The agency approved Lyfgenia from bluebird bio, and a separate treatment called Casgevy by partners Vertex Pharmaceuticals and CRISPR Therapeutics. Both the therapies were … Read more